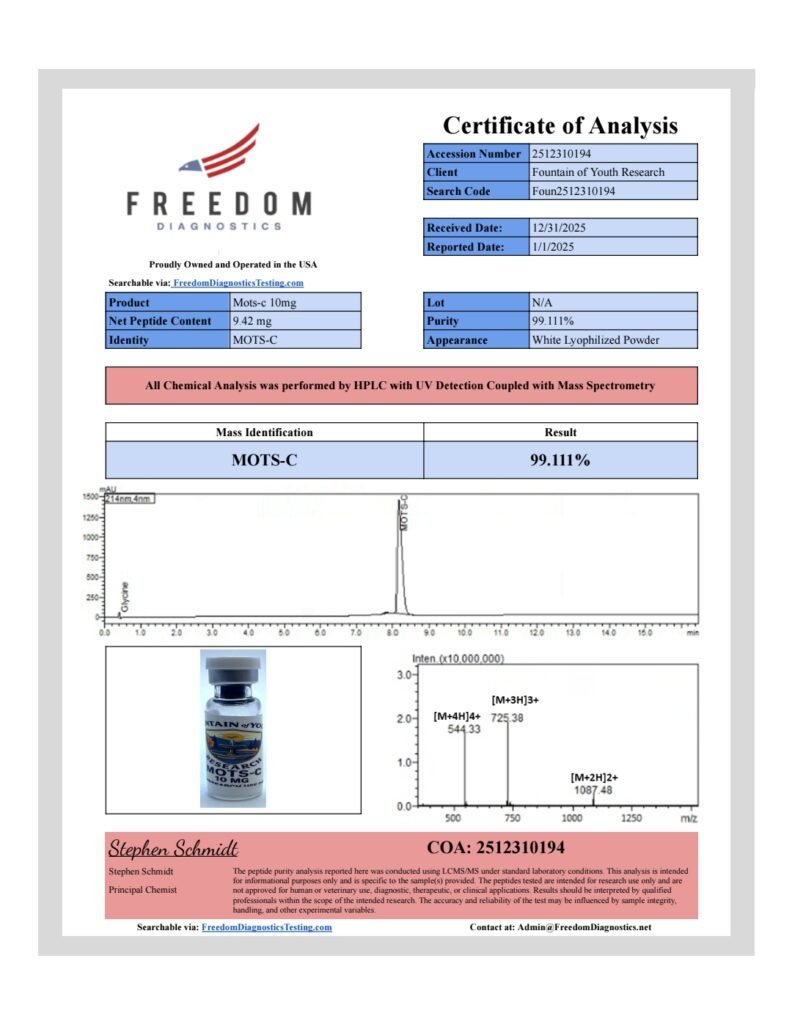
MOTS-C 10 mg
$110.00
MOTS-c is a mitochondria-derived research peptide consisting of 16 amino acids (Met-Arg-Trp-Gln-Glu-Met-Gly-Tyr-Ile-Phe-Tyr-Pro-Arg-Lys-Leu-Arg). It is encoded within the mitochondrial 12S rRNA gene and has been shown in preclinical studies to influence cellular metabolism through activation of AMPK and regulation of mitochondrial homeostasis. As a result, MOTS-c is commonly studied in metabolic and aging research focused on mitochondrial signaling, stress adaptation, and energy regulation pathways.
For research use only. Not for human consumption.
MOTS-c Overview
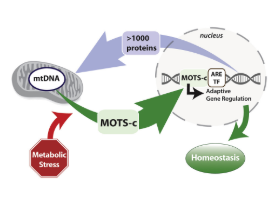
MOTS-c is a short peptide encoded within the mitochondrial genome and is part of the broader family of mitochondrial-derived peptides (MDPs). MDPs have recently been recognized as bioactive hormones that play key roles in mitochondrial signaling and energy regulation. While initially believed to act only within mitochondria, emerging research shows that many MDPs are active in the cell nucleus and some enter the bloodstream to exert systemic effects.
MOTS-c, in particular, has been identified as a critical regulator of metabolism, weight management, exercise capacity, longevity, and disease-related processes such as osteoporosis. It has been detected both in the cell nucleus and in circulation, confirming its status as a naturally occurring hormone. Due to its broad physiological impact and therapeutic potential, MOTS-c has been a focus of intensive research over the past five years.
MOTS-c Structure
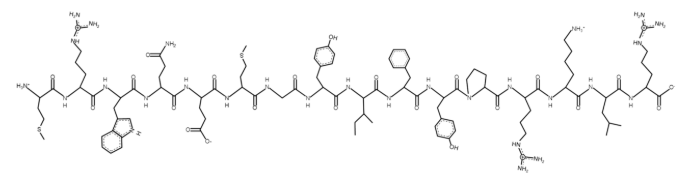
Sequence: Met-Arg-Trp-GIn-Glu-Met-Gly-Tyr-lle-Phe-Tyr-Pro-Arg-Lys-Leu-Arg
Molecular Formula: C101H152Nz8 022S2
Molecular Weight: 2174.64 g/mol
PubChem SID: 255386757
Synonyms: Mitochondrial open reading frame of the 12S RNA-c, MT-RNR1
MOTS-c Research
Muscle Metabolism
Mouse studies indicate that MOTS-c can counteract age-related insulin resistance in skeletal muscle, enhancing glucose uptake. This effect is achieved by improving muscle responsiveness to AMPK activation, which subsequently increases the expression of glucose transporters. Notably, this mechanism operates independently of the insulin pathway, providing an alternative route for promoting glucose uptake when insulin is insufficient or ineffective. The overall outcomes include improved muscle function, enhanced muscle growth, and reduced functional insulin resistance.
Fat Metabolism
Mouse studies have shown that low estrogen levels can lead to increased fat accumulation and impaired adipose tissue function, elevating the risk of insulin resistance and diabetes. Supplementation with MOTS-c, however, has been found to enhance brown fat activity, reduce adipose tissue accumulation, and prevent adipose dysfunction and the inflammation that often precedes insulin resistance.
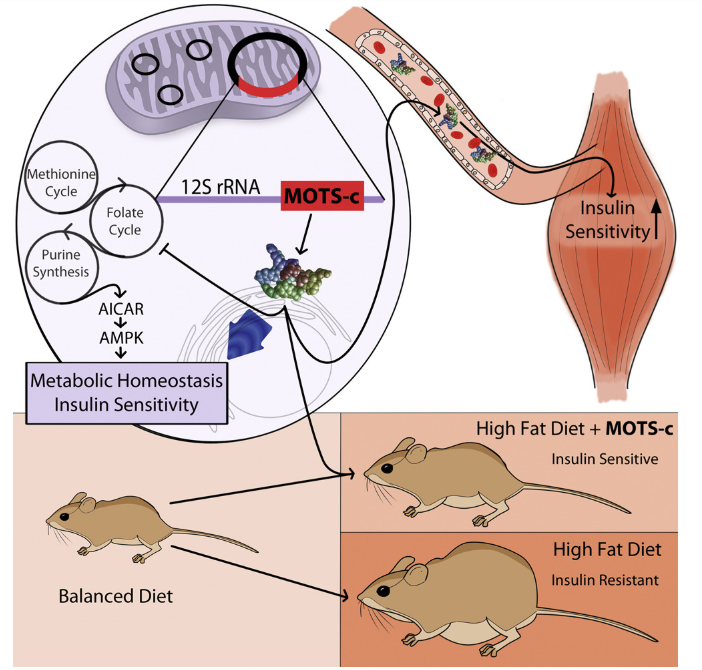
Much of MOTS-c’s effect on fat metabolism appears to be mediated through activation of the AMPK pathway. This energy-sensing pathway is triggered when cellular energy levels are low and promotes the uptake of glucose and fatty acids for metabolism. AMPK activation is also a key mechanism in ketogenic diets, such as the Atkins diet, which support fat utilization while preserving lean body mass. MOTS-c further influences metabolism by targeting the methionine-folate cycle, increasing AICAR levels, and activating AMPK.
Recent research indicates that MOTS-c can exit the mitochondria and enter the cell nucleus, where it regulates nuclear gene expression. Under metabolic stress, MOTS-c has been shown to modulate genes involved in glucose restriction and antioxidant responses, highlighting its role as a regulator of both cellular and systemic metabolism.
Evidence from mouse studies suggests that MOTS-c plays a key role in regulating lipid metabolism, particularly in the context of obesity. The peptide influences sphingolipid, monoacylglycerol, and dicarboxylate pathways, downregulating these processes while promoting beta-oxidation, which helps prevent fat accumulation. Many of these effects are likely mediated by MOTS-c’s activity in the cell nucleus.
Research on MOTS-c has led to a new hypothesis regarding fat deposition and insulin resistance that is gaining attention in the scientific community. According to this model, mitochondrial dysfunction in fat metabolism reduces fat oxidation, resulting in elevated circulating lipid levels. The body compensates by increasing insulin production to clear these lipids from the bloodstream. Over time, this compensatory response contributes to increased fat storage and induces a homeostatic shift, ultimately leading to chronic insulin resistance. MOTS-c’s regulatory effects may therefore offer a novel avenue for intervening in the mechanisms underlying obesity and diabetes.
Insulin Sensitivity
Studies examining MOTS-c levels in insulin-sensitive and insulin-resistant individuals indicate that the peptide is associated with insulin sensitivity primarily in lean individuals. This suggests that MOTS-c may play a role in the development of insulin resistance, rather than in its maintenance. Researchers propose that MOTS-c could serve as a biomarker for identifying pre-diabetic lean individuals, with changes in its levels acting as an early warning of potential insulin insensitivity.
Supplementation with MOTS-c in this context may help prevent the onset of insulin resistance and reduce the risk of developing diabetes. While preclinical studies in mice have shown promising results, further research is needed to fully understand the effects of MOTS-c on insulin regulation and its potential therapeutic applications.
Osteoporosis
MOTS-c plays a significant role in bone health by supporting type I collagen synthesis in osteoblasts. Studies in osteoblast cell lines have shown that MOTS-c regulates the TGF-β/SMAD signaling pathway, which is crucial for osteoblast survival and function. By enhancing osteoblast viability, MOTS-c contributes to stronger collagen production, thereby improving bone strength and structural integrity.
Further research in osteoporosis models has demonstrated that MOTS-c also promotes the differentiation of bone marrow stem cells through the same TGF-β/SMAD pathway. This action directly stimulates osteogenesis, or the formation of new bone. Consequently, MOTS-c not only protects existing osteoblasts but also supports their development from stem cells, highlighting its dual role in maintaining and enhancing bone health.
Longevity
Research on MOTS-c has identified a specific genetic variation associated with increased longevity in certain human populations, notably among the Japanese. This variation involves the substitution of lysine with glutamate at position 14 of the peptide. While the exact functional consequences of this substitution are not yet fully understood, the chemical differences between lysine and glutamate suggest that it likely alters both the structure and activity of MOTS-c. This variant appears to be exclusive to individuals of Northeast Asian ancestry and is believed to contribute to the exceptional longevity observed in this population.
Dr. Changhan David Lee, a researcher at the USC Leonard Davis School of Gerontology, emphasizes that mitochondrial biology is central to extending both lifespan and healthspan in humans. As the mitochondria are the primary metabolic organelles, they are strongly implicated in aging and age-related diseases. Until recently, dietary restriction was the most reliable method for modulating mitochondrial function to influence longevity. Peptides like MOTS-c, however, offer a potential means to directly and more effectively enhance mitochondrial function, opening new avenues for promoting healthy aging.
Heart Health
Human studies have shown that lower circulating levels of MOTS-c are associated with increased endothelial dysfunction. Endothelial cells, which line blood vessels, are essential for regulating blood pressure, clotting, and plaque formation. While animal studies in rats indicate that MOTS-c does not directly alter blood vessel responsiveness, it appears to enhance endothelial sensitivity to signaling molecules such as acetylcholine. Supplementation with MOTS-c in these models has been shown to improve endothelial function and enhance both microvascular and epicardial blood vessel performance.
MOTS-c is not unique among mitochondrial-derived peptides (MDPs) in influencing cardiovascular health. Research suggests that several MDPs protect cardiac cells from stress and inflammation, and dysregulation of these peptides may contribute to the development of cardiovascular disease. They may also play roles in reperfusion injury and in maintaining endothelial function.
In preclinical studies, MOTS-c has demonstrated minimal side effects, low oral bioavailability, and excellent subcutaneous bioavailability in mice. Dosages in mice do not directly translate to humans. MOTS-c is intended strictly for educational and scientific research purposes and is not approved for human consumption. Only licensed researchers should purchase and use MOTS-c.
You May Be Interested In...
Out of stock